Published February 2021
In the ongoing global fight against COVID-19, The Ottawa Hospital is proud to help fill Canada’s critical needs in vaccine manufacturing. As one of only a handful of hospitals with the capacity to manufacture vaccines, and as the most experienced facility of its kind in Canada, our hospital is uniquely positioned to partner with Edmonton-based Entos Pharmaceuticals to help manufacture Covigenix VAX-001 in vials for Stage 1 clinical trials being conducted at the Canadian Centre for Vaccinology.
“I think it’s very important that we have the capacity to be able to produce these kinds of products, particularly vaccines in the midst of the pandemic in Canada.”
— Dr. Duncan Stewart, senior scientist in the Regenerative Medicine Program and professor at the University of Ottawa
In the News
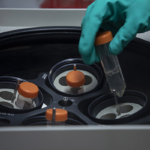
The Ottawa Hospital to produce COVID-19 vaccines for clinical trials.
Global News, Feb. 26, 2021
On This Page
Why is developing new COVID-19 vaccines important?
With several vaccines now approved and being administered in many countries around the world, some might wonder why researchers at The Ottawa Hospital are continuing to create and test potential new vaccine candidates. There are several important reasons why:









